Compute the theoretical density of ZnS given that the Zn-S distance and bond angle are 0.234 nm and - Brainly.com

Calculated electronic, transport, and bulk properties of zinc-blende zinc sulphide (zb-ZnS) - ScienceDirect
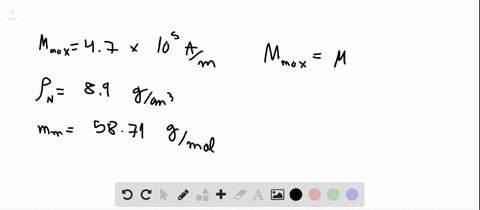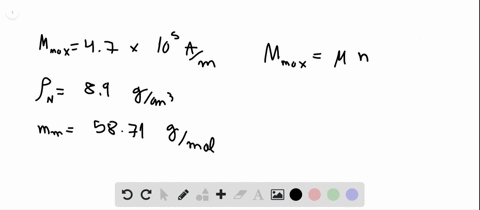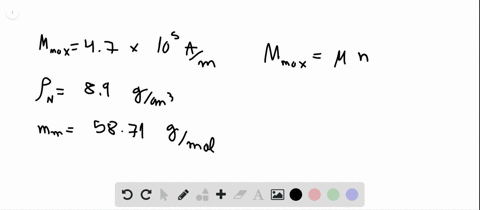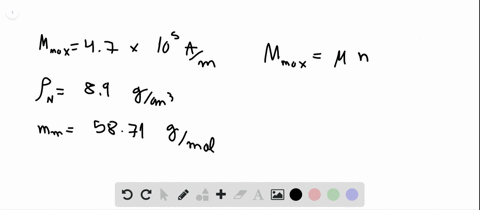
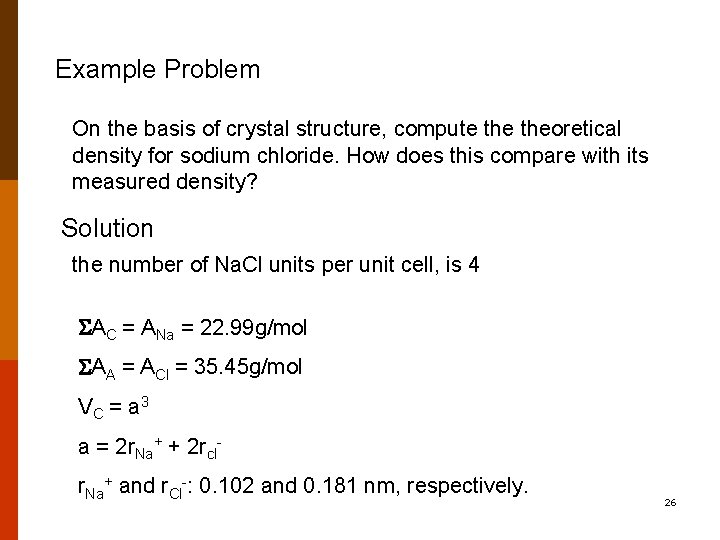
SOLVED:Compute the theoretical density of ZnS given that the \mathrm{Zn}-\mathrm{S} distance and bond angle are 0.234 \mathrm{nm} and 109.5^{\circ}, respectively How does this value compare with the measured density?

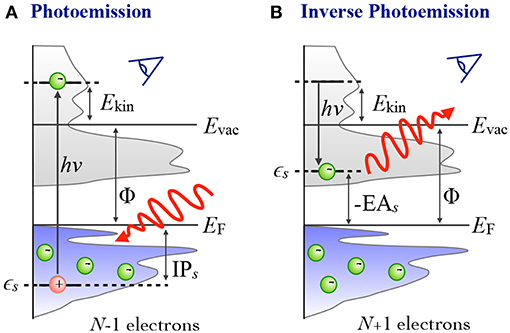
Density functional theory in the solid state | Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences

Module 6.docx - Module 6 1. The number of vacancies in some hypothetical metal increases by a factor of 6 when the temperature is increased from 958 C | Course Hero

Chemical Functionalization of ZnS: A Perspective from the Ligand–ZnS Bond Character | The Journal of Physical Chemistry C

SOLVED:Compute the theoretical density of ZnS, given that the Zn—S distance and bond angle are 0.234 nm and109.5, respectively. How does this value compare with the measured density?