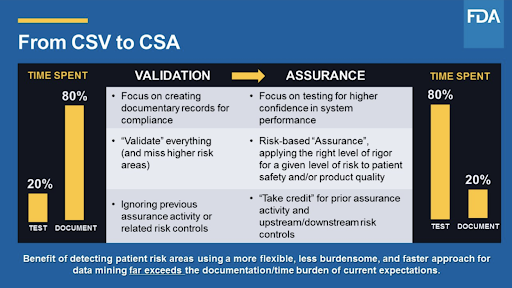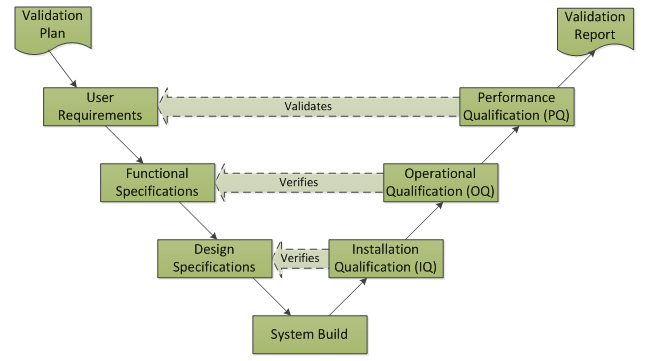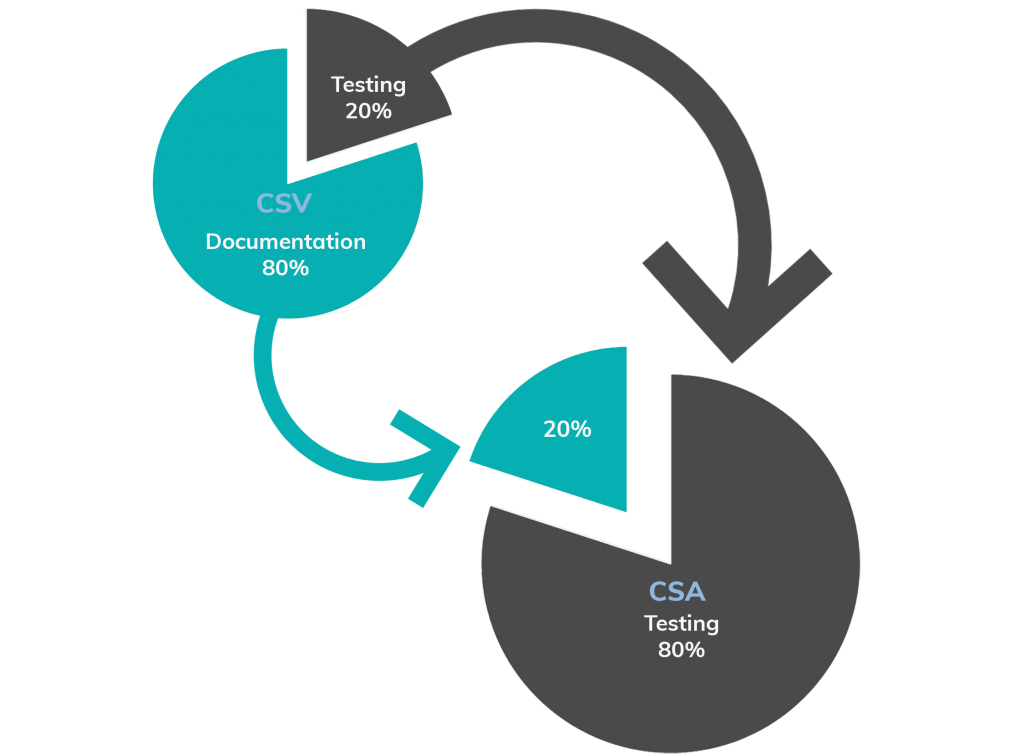
What is Computer Software Assurance (CSA) and why are the FDA transitioning from traditional Computer System Validation? - Kneat

FDA Requests Public Comment On Credibility Of Medical Device Models | MedTruth - Prescription Drug & Medical Device Safety | Informed Advocacy
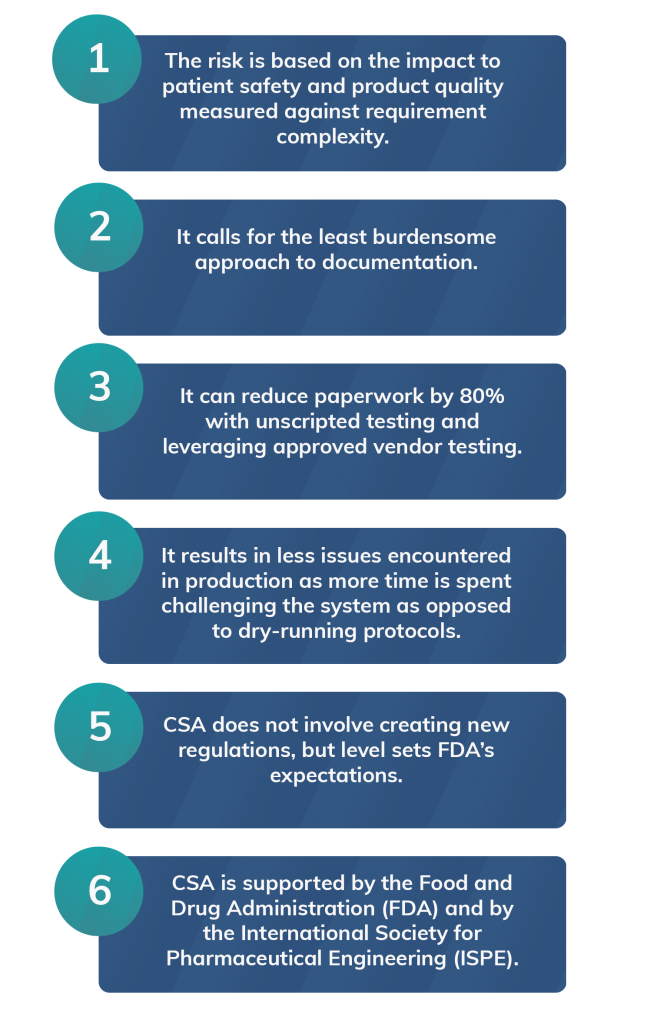
What is Computer Software Assurance (CSA) and why are the FDA transitioning from traditional Computer System Validation? - Kneat

FDA's Technology Modernization Action Plan Accelerates the Path to Enhancing and Promoting “People First” Public Health | FDA

FDA Guidance on Software Validation: Automated Process Equipment and Quality System Software | RegDesk