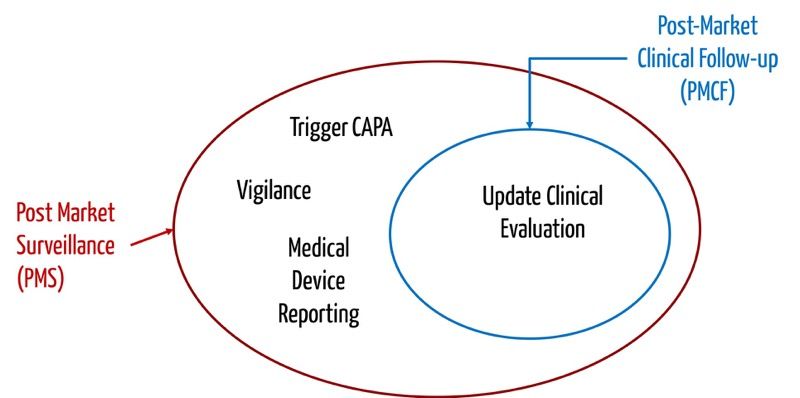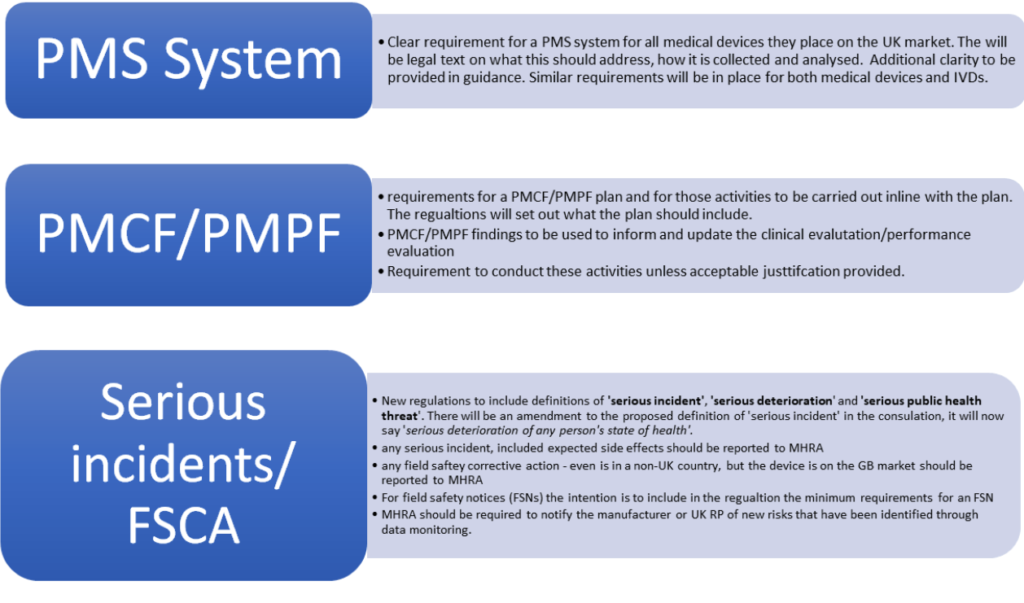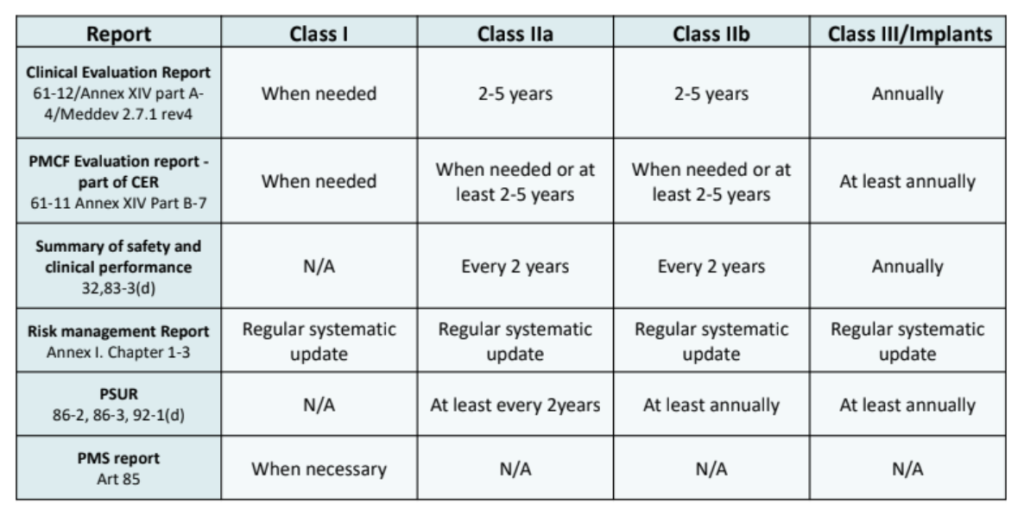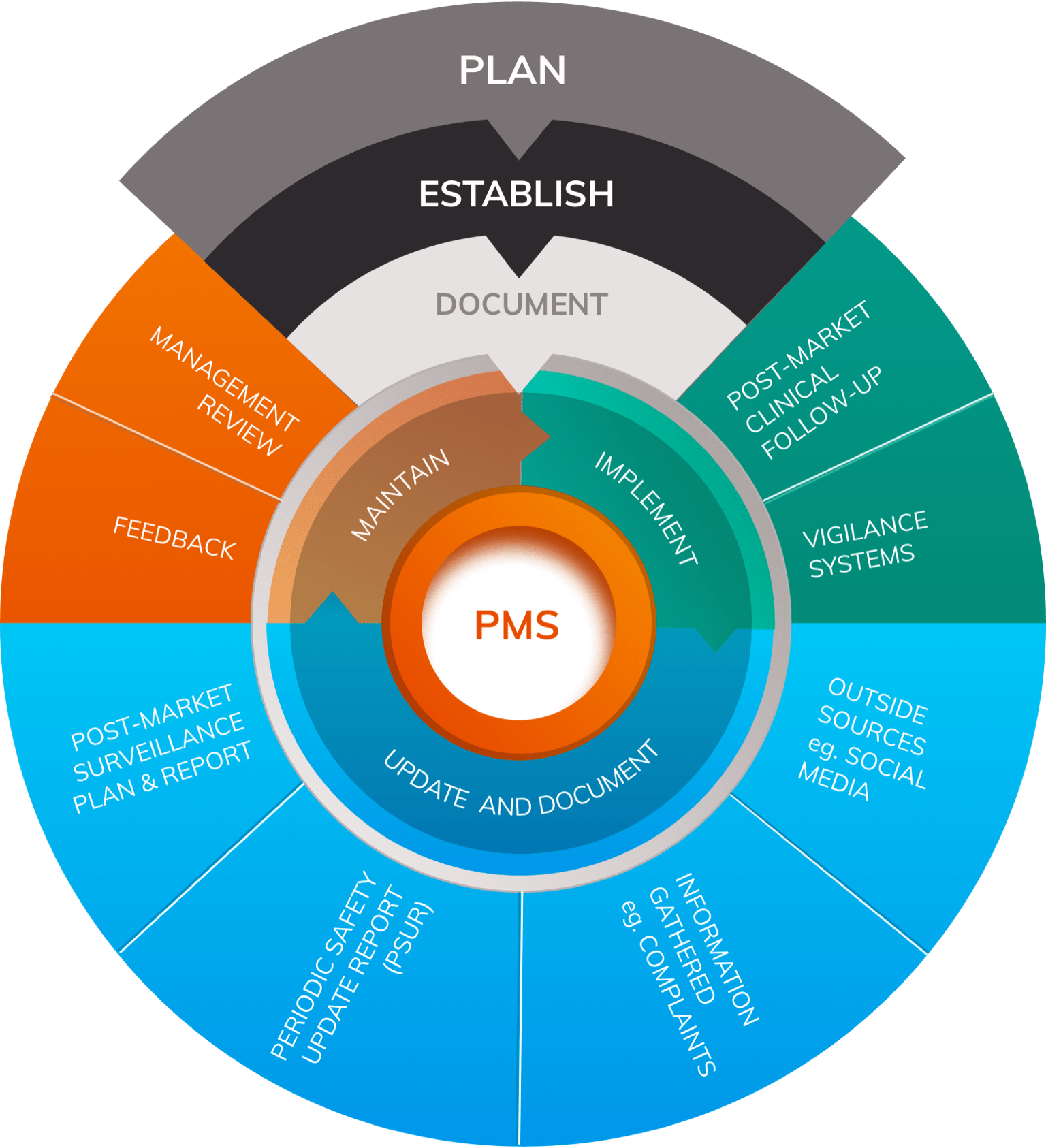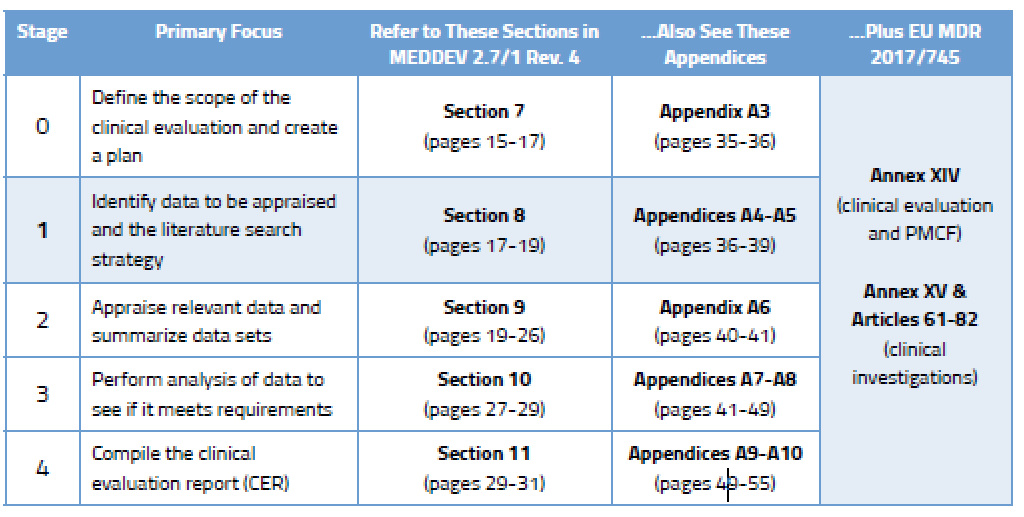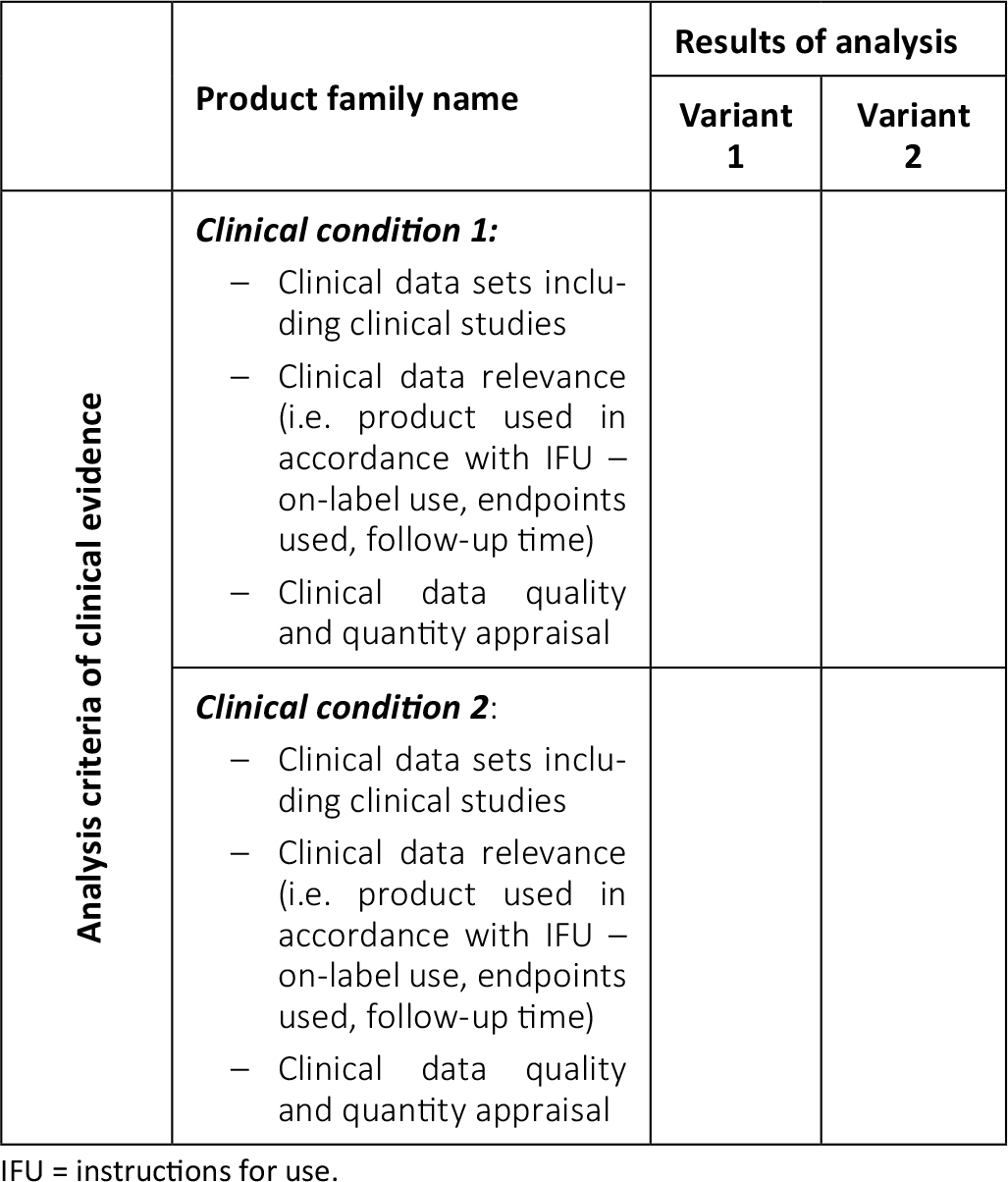
View of Getting your devices ready for MDR compliance – a clinical approach and orthopaedic device manufacturers' perspective | AboutOpen
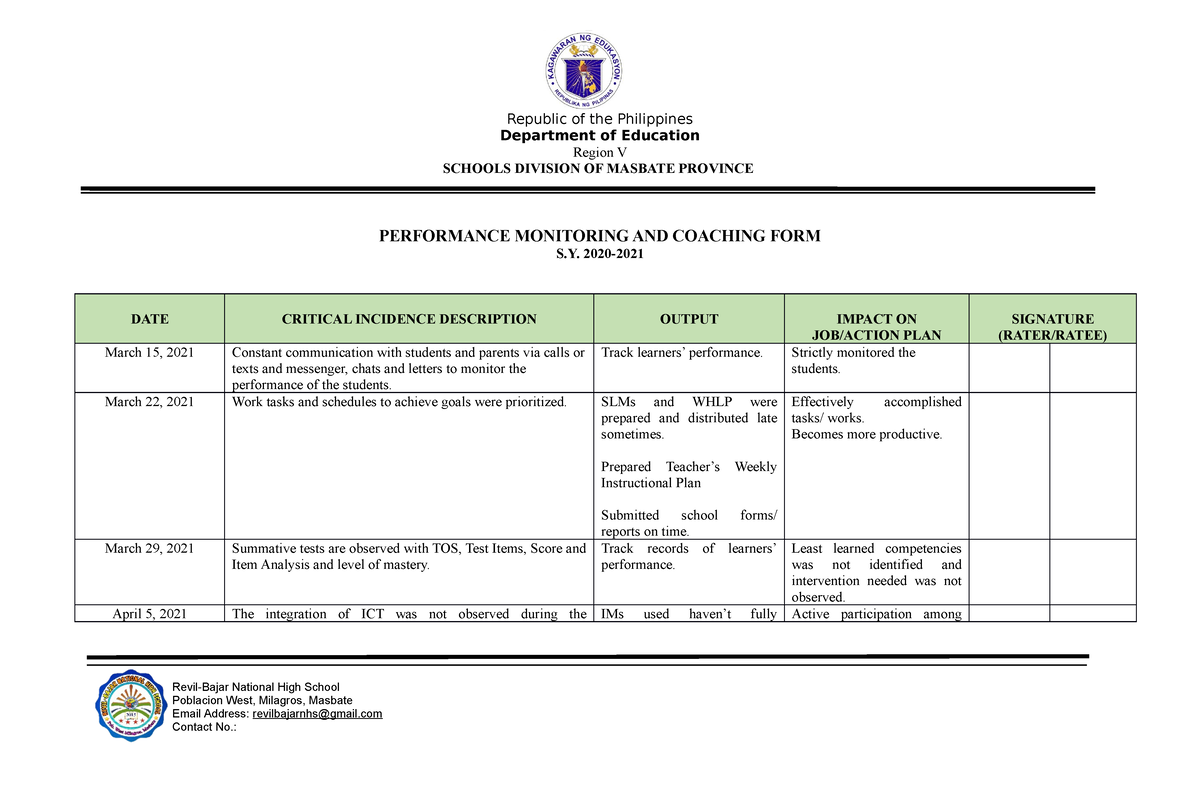
PMCF (2019-2020) - Performance Monitoring Coaching Form (2019-2020) - Republic of the Philippines - Studocu

EU Review and assessment of market surveillance activities 2014-2016 – Medical devices sector – Medical Device Expert News